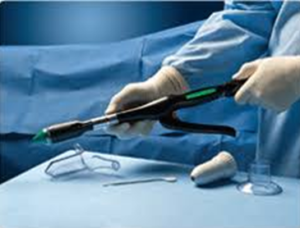
History: After a seven year review the FDA is warning doctors and patients that the malfunction or misuse of surgical staplers can result in serious injury or death. The FDA records show that more than 100,000 reports have been filed for surgical staplers since 2011 with over 10.000 serious injuries and nearly 400 patient deaths. On April 11, 2019 the FDA forced Ethicon to issue a recall of their Endo-Surgery Curved Intraluminal Staplers due to ongoing complications. These staplers were used during gastrointestinal surgeries, such as colorectal cancer or bariatric surgery, to create connections between structures. Surgical staplers and staples for internal use are also used in a wide range of surgical applications besides gastrointestinal surgeries. They are also used for gynecologic and thoracic surgeries to remove part of an organ, to cut through organs and tissues, and to create connections between structures.
Side Effects: Staple opening or malformation, tissue or organ damage, internal bleeding, infections, sepsis, leak in the closure, prolonged or repeat surgery, the need for permanent ostomy “bag, cancer recurrence after tumor removal or death
Manufacturer: Johnson & Johnson’s Ethicon